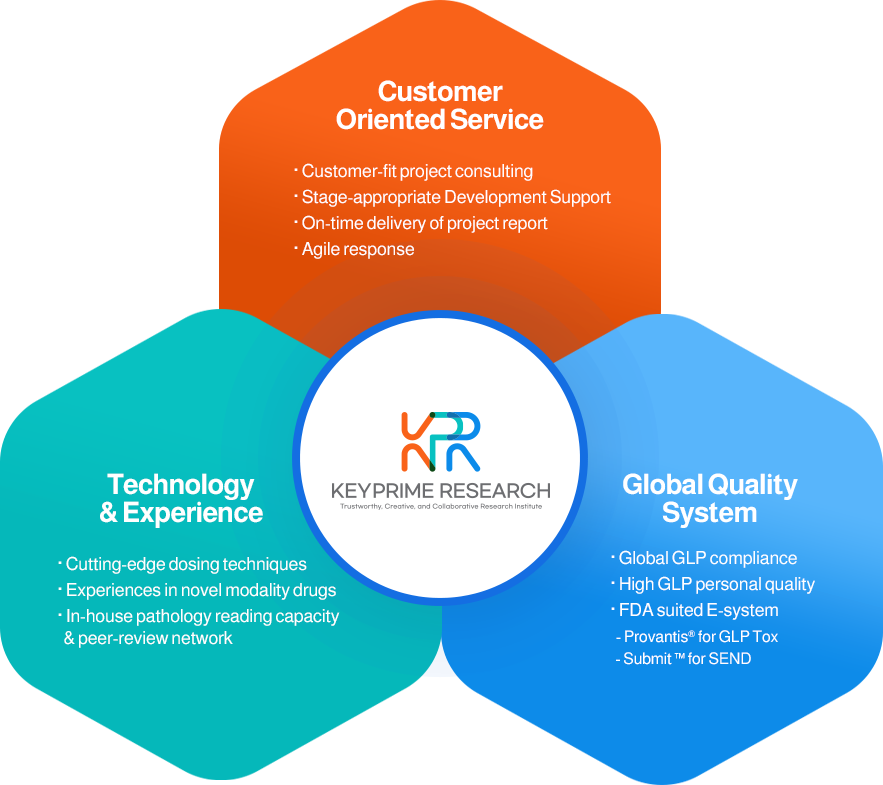
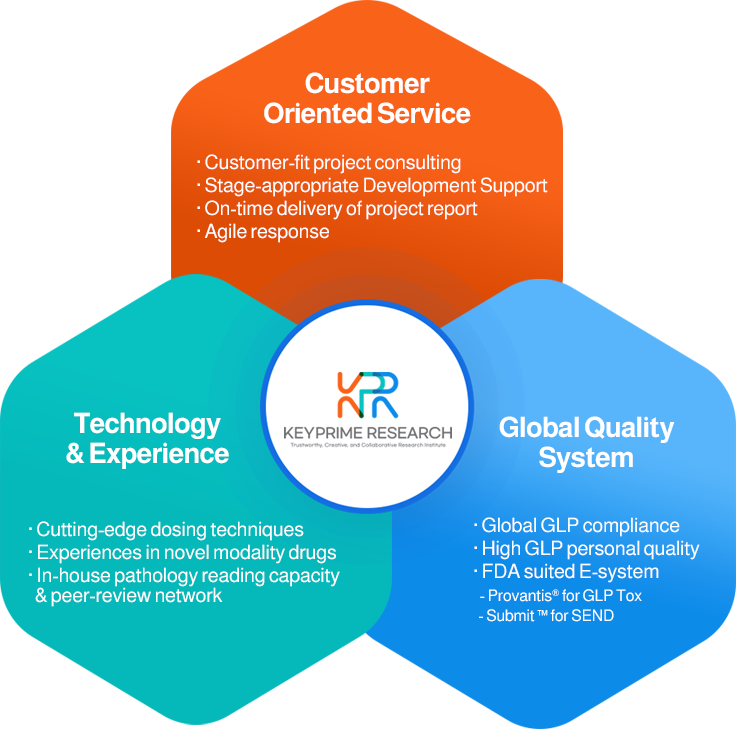
개인정보처리방침
개인정보 수집이용에 대한 동의
회원님의 소중한 개인정보는 다음과 같은 정책에 따라 수집 및 이용됩니다. ㈜키프라임리서치(이하 “회사는”)는 해당 목적에 관련되는 개인정보만을 수집하며, 수집된 정보를 투명하고 안전하게 보호 관리할 것을 약속합니다. 이에 개인정보 수집및 이용에 대한 동의를 구합니다.
개인정보의 수집·이용 목적
본 문의에 대한 문의에 대한 정보가 보다 정확한 답변을 위해 수집됩니다.
수집항목
- 필수항목: 성함, 휴대폰번호, 이메일
- 선택항목: 문의내용
보유이용기간
원칙적으로, 개인정보 수집 및 이용목적이 달성된 후에는 해당 정보를 지체 없이 파기합니다. 단, 다음의 정보에 대해서는 아래의 이유로 명시한 기간 동안 보존합니다.
- 보존 이유 : 회원님의 동의를 통한 정보 유지
- 보존 기간 : 회원정보 삭제 요청시까지
기타
현 개인정보처리방침은 2024년 07월 01일부터 적용되며, 법령, 정책 또는 보안기술의 변경에 따라 내용의 추가, 삭제 및 수정이 있을 시에는 변경사항 시행일의 7일 전부터 홈페이지를 통해 고지 할 것입니다.
- 시행일자 : 2024년 07월 01일
- 개인정보처리방침 버전 : version 1.0.0


 ENG
ENG